Last week, the U.S. Food and Drug Administration (FDA) approved the Impella heart pump to treat patients with severe heart failure (cardiogenic shock) after a heart attack or heart surgery. This is a major step and advancement in technology to help these very sick patients who need additional temporary support while the heart recovers its own function, or before they undergo further advanced treatments such as implantation of an artificial heart (Ventricular Assist Device [VAD]) or be considered for heart transplantation.
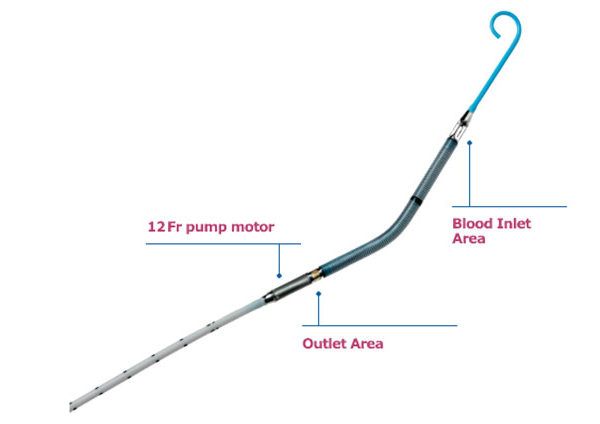
The Impella device is a temporary heart support device that can be simply delivered via small tubes or catheters from the leg or arm artery to the main pumping chamber of the heart without need for a major operation. The pump allows fresh blood from the heart (left ventricle) to be ejected out to the rest of the body in addition to the heart ‘s own pump function. This additional support can allow the heart to recover or provide time for the cardiologists and patients to consider future more complex treatment options. The Impella device has been approved and used for patients who need complex and high risk angioplasty on their coronary or heart arteries, but its approval for use for patients with severe heart dysfunction is an important step and provides cardiologists another life-saving tool in their armamentarium of devices. It is important to note that these devices carry their own but small risks and need a specialized team of cardiologists and critical care nurses to manage these sick patients.
CardioVisual can help cardiologists and other health care providers supply information about Impella to their patients, along with videos of other procedures and treatments that may be involved with this procedure to help patients understand better especially when dealing with such complex procedures during a stressful time. CardioVisual app provides a comprehensive library of short yet thorough videos that help patients understand complicated cardiac treatments and procedures.